New gene therapy offers hope for Huntington’s disease, potentially increasing quality of life significantly. Trial results indicate a 75% reduction in disease progression after three years, showcasing the treatment’s transformative potential, as highlighted by experts from uniQure.
Huntington’s disease is a rare, inherited neurodegenerative disorder that eventually damages brain cells, resulting in movement challenges, cognitive decline, and psychiatric issues. Symptoms usually emerge in an individual’s 30s or 40s, which gets worse over time, and often lead to death within 10 to 30 years.

What is Huntington’s disease?
Huntington’s disease is caused by an error in part of our DNA called the huntingtin gene.
If one of your parents has Huntington’s disease, there’s a 50% chance that you will inherit the altered gene and will eventually develop Huntington’s too.
This mutation turns a normal protein needed in the brain – called the huntingtin protein – into a killer of neurons.
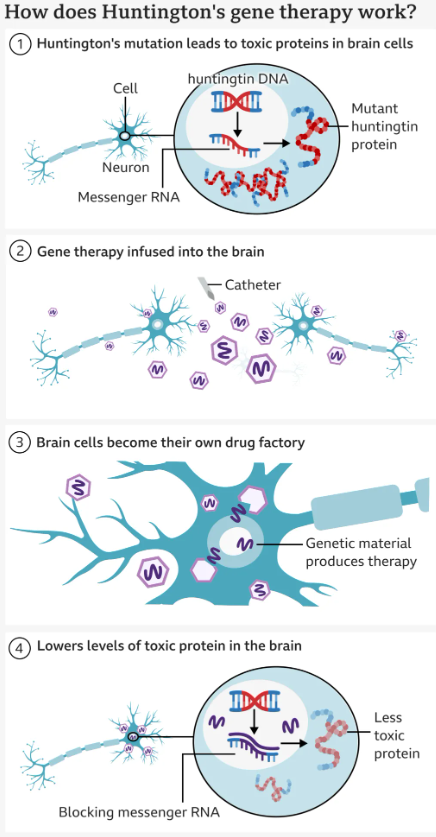
The goal of the treatment is to reduce levels of this toxic protein permanently, in a single dose.
The therapy uses cutting edge genetic medicine combining gene therapy and gene silencing technologies.
Physical symptoms in Huntington’s disease
Physical symptoms can involve slurred speech and difficulties with swallowing, eating, and walking. Due to these challenges, many individuals with HD may experience weight loss caused by trouble feeding, choking and frequent chest infections. Other common symptoms include insomnia (difficulty sleeping), low energy, fatigue, and seizures. As the disease progresses, the person may become confined to a bed or wheelchair.
‘75% slowing of clinical progression’
The trial results, involving 29 patients, were announced in a statement by the company uniQure, though the full findings have not yet been published for peer review by independent experts. According to the data, three years after undergoing surgery, patients showed an average 75% reduction in disease progression, based on a combined measure of cognitive abilities, motor skills, and daily functioning, as per a BBC report.
According to Prof. Sarah Tabrizi, this means that the deterioration typically seen over one year would now take around four years, potentially offering patients many more years of high-quality life. The breakthrough treatment involves a form of gene therapy delivered through a delicate brain surgery lasting between 12 and 18 hours, the report said.
“We never in our wildest dreams would have expected a 75% slowing of clinical progression,” BBC quoted Tabrizi as saying.
The chief medical officer at uniQure, Dr Walid Abi-Saab, stated he was “incredibly excited” about what the outcomes mean for families and mentioned that the treatment had “the potential to fundamentally transform” Huntington’s disease.
A Promising One-Time Treatment
An experimental gene therapy from UniQure has highlighted remarkable outcomes in slowing Huntington’s disease. As per the study results reported Wednesday, revealed that the therapy slowed disease progression by 75% after three years, making a possible step toward the initially approved genetic treatment for the rare condition. For the people living with Huntington’s disease, an effective, one-time therapy that gradually slows the loss of muscle control and cognition in mid-life could preserve years of quality bonds and gainful employment that would usually be lost to the illness.
How AMT-130 Works
The therapy, called AMT-130, includes a single-dose gene treatment offered through 12 to 18 hours of neurosurgery. A renewed virus is infused into two brain areas, the caudate nucleus and putamen—where it carries DNA designed to minimize production of the toxic huntingtin protein that damages neurons. Trial data, released but not yet peer-reviewed, emphasized that patients faced a 75% slowing of disease advancement three years after treatment, measured via combined cognitive, motor, and daily living evaluations.Usually , this level of reduction would happen in just one year.
The trial also showcased biological proof of benefit, as levels of neurofilaments, proteins in spinal fluid that elevates as brain cells die, were lesser than at the study’s beginning. Prof. Ed Wild, consultant neurologist at University College London Hospitals, cited, “This is the result we’ve been waiting for. The magnitude of the effect is breathtaking.”

Prof. Sarah Tabrizi, director of the University College London Huntington’s Disease Centre, explained the outcomes as “spectacular,” adding, “We never in our wildest dreams would have expected a 75% slowing of clinical progression.” AMT-130 was normally well-tolerated, with no recent critical side effects reported since late 2022. The treatment is anticipated to be long-lasting, as brain cells do not regenerate like other tissues. Some patients had mild inflammation in regard to the viral delivery system, resulting in headaches and confusion, which was resolved with steroids.
Result of the Trial
The study included 29 patients with symptomatic Huntington’s disease. Many reported significant improvements: one person who had retired on medical grounds resumed to work, while others maintained mobility longer than expected. UniQure plans to submit a marketing application to the U.S. Food and Drug Administration in early 2026, targeting to introduce the treatment later that year if approved.
Understanding Huntington’s Disease
Huntington’s disease is the result of mutation in the huntingtin gene. Affected parents’ children have a 50% chance of inheriting the disorder. Symptoms generally appear in mid-adulthood, and the condition is usually fatal within two decades. In the UK, US, and Europe, almost 75,000 people are impacted, with hundreds of thousands more carrying the faulty gene.
The breakthrough therapy provides real hope for a condition that has long had no cure, possibly transforming lives for thousands of patients globally.
Also Read :
Joshua Jahn Identified in Dallas ICE Facility Shooting – What We Know So Far
Trump’s UN Speech Sparks Debate on U.S. Role in the World Order
Italian Cinema Queen and star of The Leopard Claudia Cardinale Dies At 87